Navigating the 2026 "CMC Cliff" for AAV Gene Therapy
January 15, 2026
Adeno-associated virus (AAV) gene therapy is one of the most promising, yet most closely scrutinized modalities reviewed by the FDA. As we settle into early 2026, the AAV gene therapy sector has entered a defining moment. The narrative has shifted from “can we cure it?” to “can we make it consistently?” For regulatory marketing professionals and biotech stakeholders, the data is unequivocal: clinical efficacy is no longer the primary hurdle for approval. The hurdle is industrialization.
This report analyzes the “CMC Cliff,” a phenomenon where promising programs with robust clinical data suddenly stall due to manufacturing immaturity or facility unreadiness. With the FDA’s January 11, 2026, guidance introducing a landmark, but complex flexible framework, sponsors now face a paradox: the Agency is willing to accept risk-based justifications for early-phase release criteria, but only if they are supported by state-of-the-art analytical maturity.
The Regulatory Shift – The January 2026 Guidance
On January 11, 2026, the FDA formalized its stance on manufacturing with the guidance “Flexible Requirements for Cell and Gene Therapies to Advance Innovation.” This announcement is a lifeline for AAV startups facing the “Valley of Death,” but it requires careful interpretation to avoid compliance traps.
GMP Phase-In: “Permissive” Does Not Mean “Uncontrolled”
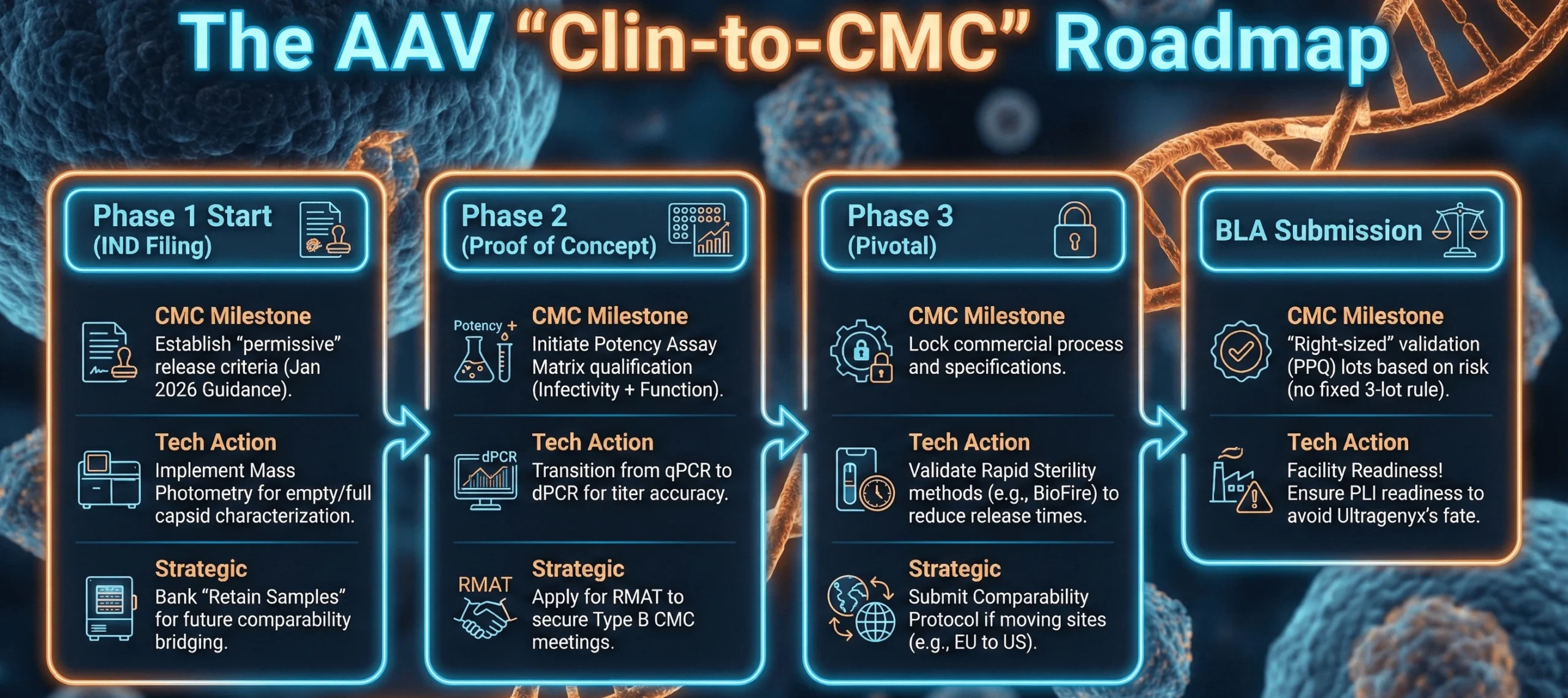
The guidance clarifies that manufacturers are not expected to fully comply with 21 CFR Part 211 (Good Manufacturing Practices) prior to Phase 2/3 trials. This allows early-stage sponsors to operate with “permissive product quality release acceptance criteria” during Phase 1.
Strategic Implication: You can save capital on GMP documentation systems in Phase 1, but you cannot compromise on safety testing. The flexibility applies to administrative GMP compliance, not product safety (e.g., sterility, mycoplasma, and adventitious agents must still be compendial or equivalent).
Validation “Right-Sizing” for Rare Diseases
The agency clarified that there is no fixed requirement for three Process Performance Qualification (PPQ) lots. The number of validation lots can be scientifically justified based on overall process understanding and risk.
The AAV Context: For ultra-rare indications where a single 200L bioreactor run might treat the entire patient population, this allows for a “bracketed approach” to validation. MeiraGTx successfully utilized this strategy in late 2025, aligning with the FDA on an expedited CMC PPQ package for their AAV-hAQP1 program.
Regulatory flexibility must be tailored for cell and gene therapies. These are common-sense reforms that will address the unique characteristics of cell and gene therapies and foster more innovation.— FDA Press Announcement, January 11, 2026
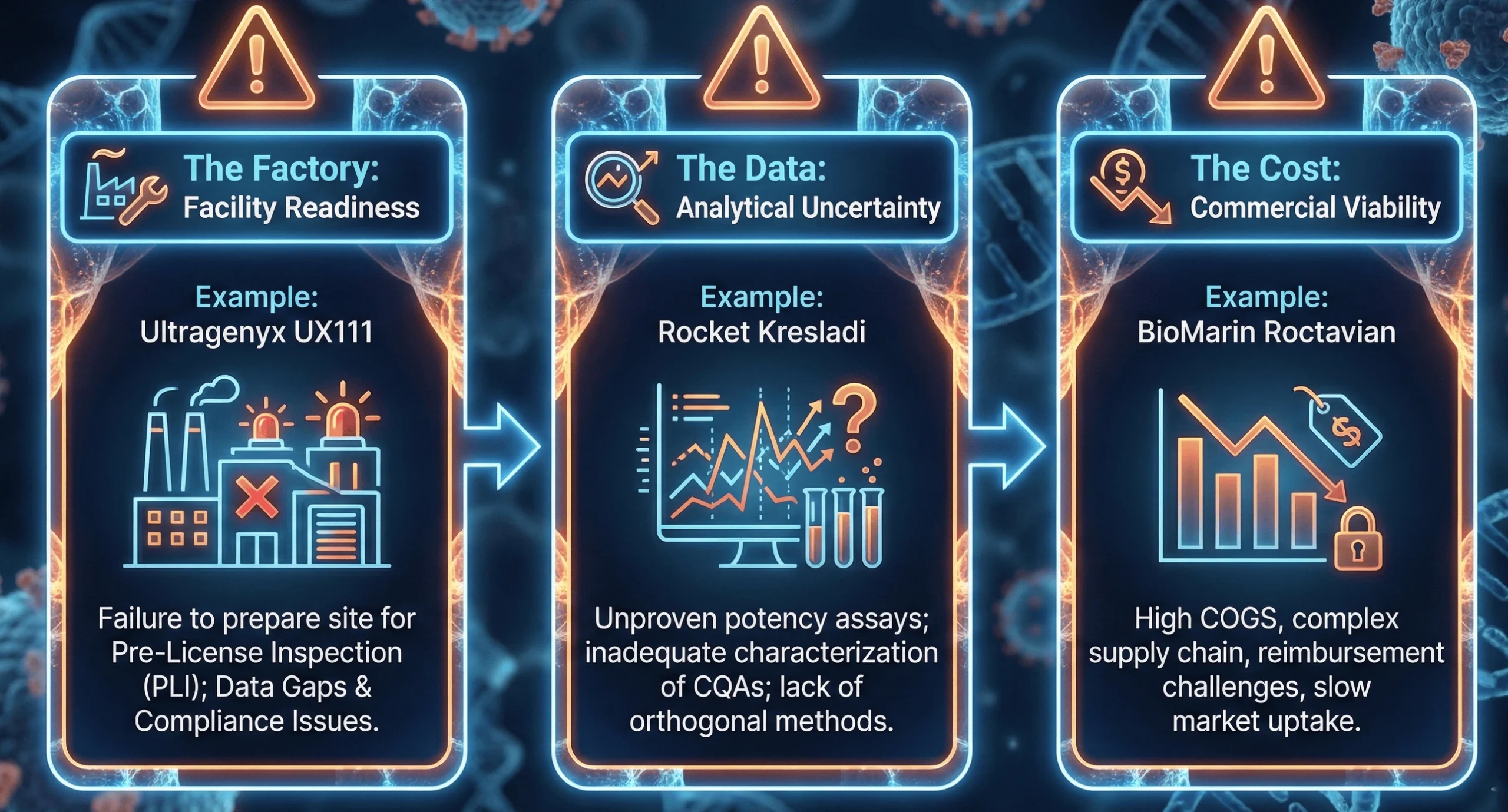
Anatomy of an AAV Failure – Lessons from the Trenches
The “CMC Cliff” is littered with cautionary tales. By analyzing recent CRLs and withdrawals, we can construct a taxonomy of failure modes that current AAV sponsors must avoid.
The Technical Battleground – Critical Quality Attributes (CQAs)
To avoid the fate of UX111, sponsors must master specific technical domains. The FDA’s focus has shifted from “platform” justifications to product-specific resolution.
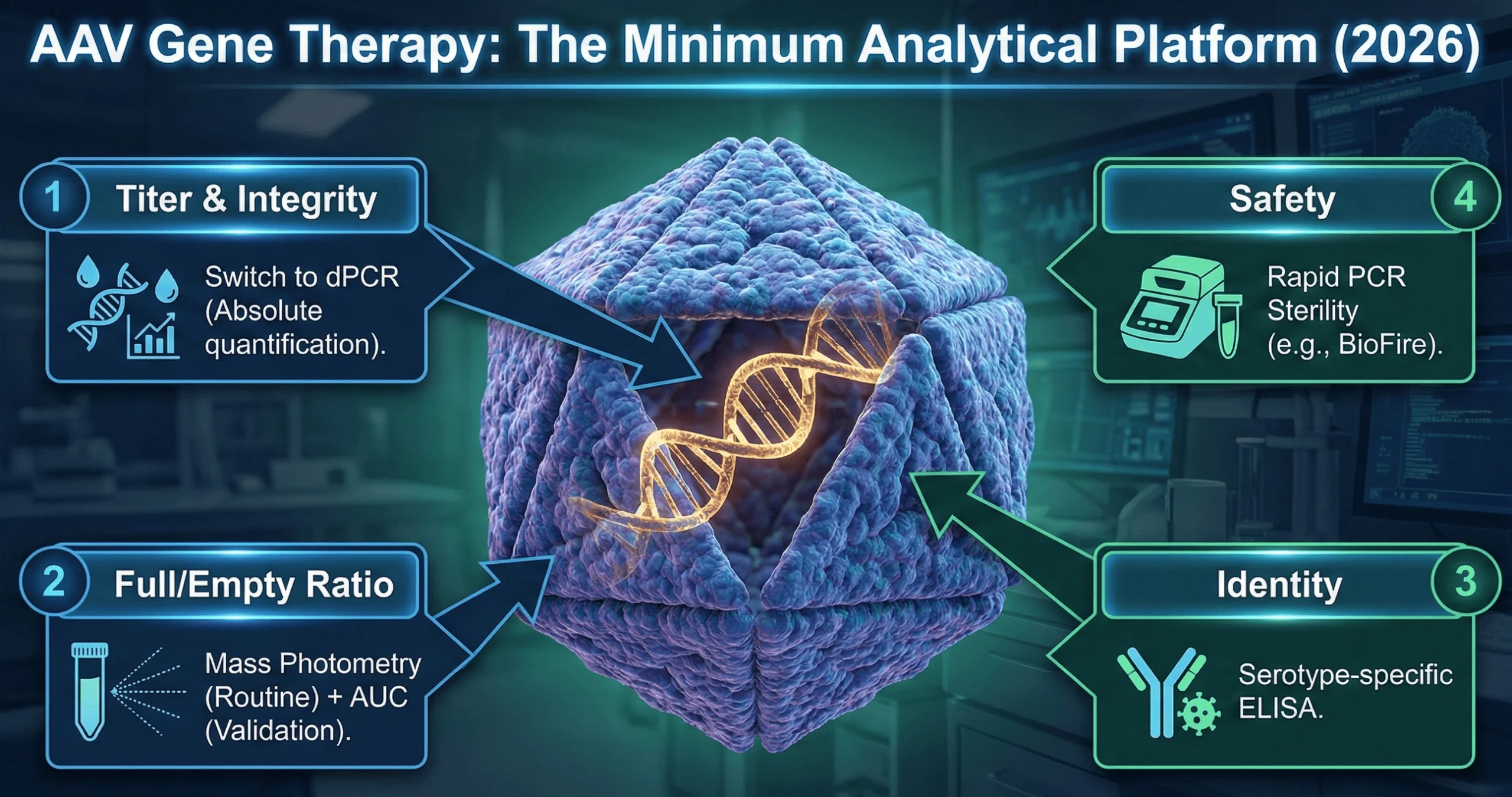
1. Genome Integrity: The “Digital” Shift
Quantifying the viral titer (vector genomes per mL) is critical for dosing. The industry is undergoing a wholesale shift from quantitative PCR (qPCR) to Digital PCR (dPCR).
- Why dPCR? It offers absolute quantification without the need for a standard curve, making it more precise and less susceptible to inhibitors found in complex formulations.
- The Risk: Transitioning from qPCR to dPCR mid-development is a major comparability event. Sponsors must run bridging studies to show how the new “dPCR titer” relates to the old “qPCR titer” used in toxicology studies.
2. Empty/Full Capsid: The “Mass Photometry” Revolution
AAV vectors are plagued by “empty” capsids—viral shells with no DNA—which increase immunogenicity without therapeutic benefit. The FDA now mandates orthogonal methods (two different technologies) to quantify this ratio.
- The 2026 Standard: Mass Photometry (MP) has emerged as the new gold standard. In mid-2025, the USP recognized mass photometry in General Chapter as a key orthogonal method for AAV characterization. Unlike bulk methods, MP provides single-particle resolution, distinguishing empty, full, and—crucially—partially filled capsids, which are a dangerous impurity often missed by ELISA or simple OD ratios.
- Emerging Tech: Charge Detection Mass Spectrometry (CD-MS) is also gaining traction for its ability to resolve the mass of the encapsidated genome, detecting truncations that might otherwise look like a “full” capsid in lower-resolution assays.
3. Potency Assays: The Matrix is Mandatory
The single most consistent IND deficiency is the lack of a relevant potency assay. The FDA now demands a matrix approach that correlates three dimensions:
- Infectivity: (e.g., TCID50) – Does the virus enter the cell?
- Expression: (e.g., RT-qPCR/Western) – Is the payload delivered?
- Function: (e.g., Enzymatic activity) – Does the protein work?
In 2026, “surrogate” potency (just measuring protein mass) is increasingly rejected. The FDA wants to see a functional bioassay that mimics the physiological effect.
4. Rapid Release Testing: Speed is Safety
For AAV therapies, waiting 14-28 days for sterility (USP ) and mycoplasma (USP ) testing is a supply chain bottleneck.
- Innovation: The FDA is accepting rapid, PCR-based sterility and mycoplasma tests (e.g., BioFire, BacT/Alert) if validated against compendial standards. The University of Pennsylvania successfully implemented BioFire for rapid mycoplasma testing, reducing release times from 28 days to hours.
The Geopolitical Constraint – The 2025 Export Ban
In a move that stunned the global biotech community, the FDA announced on June 18, 2025, an immediate halt to new clinical trials involving the export of American patients’ biological samples to “hostile countries” (specifically China) for genetic engineering.
Impact on AAV Sponsors:
While explicitly targeting ex vivo cell therapies, this policy creates a “guilt by association” risk for AAV manufacturing.
- The Supply Chain Risk: If your AAV vector or critical plasmids are manufactured in China, regulators are applying heightened scrutiny regarding data security and “biomedical integrity.”
- Strategic Action: Repatriate supply chains. Foreign sponsors targeting the US market must prioritize US or “friendly nation” CDMOs for their pivotal material to avoid the risk of a “national security” clinical hold.
Strategic Action Plan for 2026
- Leverage the RMAT Advantage – Regenerative Medicine Advanced Therapy (RMAT) designation is the most powerful tool for CMC alignment.
Action: Use RMAT “Type B” meetings specifically to resolve CMC hurdles, not just clinical ones.
Success Story: MeiraGTx used RMAT interactions to align on their potency assay and comparability strategy before filing their BLA, de-risking the entire program.
- Build a “Comparability Bank”
Action: Retain aggressive quantities of drug substance from every clinical lot. Store at -80°C. You cannot prove comparability if you have used up all your Phase 1 material.5
- Invest in “Smart” Analytics Early
Action: Adopt dPCR and Mass Photometry in Phase 1/2. The cost of the instruments is negligible compared to the cost of a clinical hold. Generating high-resolution data early creates a “data moat” that defends your product quality against regulatory questioning.6
- Use the January 2026 Flexibility Wisely
Action: Use the new guidance to delay full cGMP compliance for documentation and validation lots but maintain full cGMP standards for sterility and safety testing. Save money on the paperwork, not the pipette work.
A visual guide to aligning your AAV CMC strategy with clinical milestones to avoid a “CMC Cliff” at BLA.
How Can BLA Regulatory Help?
To successfully navigate the 2026 “CMC Cliff,” BLA Regulatory serves as an expert extension of your team, providing end-to-end strategic and technical support for AAV gene therapy programs. Led by former FDA veterans, we translate complex regulatory expectations into actionable development plans, from establishing “permissive” early-phase release criteria under the January 2026 guidance to performing rigorous regulatory gap analyses. Our proactive approach ensures sponsors are fully prepared for critical agency interactions, leveraging RMAT designations for CMC alignment and conducting mock audits to ensure your facility is Pre-License Inspection (PLI) ready. By integrating this high-level regulatory intelligence, BLA Regulatory de-risks your submission and secures a more efficient path to market approval.
References
- FDA Guidance for Industry: Flexible Requirements for Cell and Gene Therapies to Advance Innovation (January 2026) — The definitive 2026 framework allowing for phase-appropriate GMP and “permissive” early-stage release criteria.
- FDA Guidance for Industry: CMC Information for Human Gene Therapy INDs — Core requirements for documenting manufacturing and quality control for gene therapy applications.
- FDA Draft Guidance: Testing of AAV Vector-Based Gene Therapy Products for Empty Capsids — Specific expectations for quantifying empty/full ratios using orthogonal methods.
- FDA Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products — Outlines the “matrix approach” required to demonstrate biological function.
- USP General Chapter <1067>: Best Practices for the Manufacture and Quality Control of Recombinant AAV Gene Therapy Products — Established mass photometry as a key orthogonal method for AAV characterization in 2025.
- FDA Geopolitical Directive: Immediate Halt to Biological Sample Export for Genetic Engineering (June 18, 2025) — The policy requiring repatriated supply chains and heightened scrutiny for foreign manufacturing.
- Ultragenyx Corporate Announcement: Receipt of Complete Response Letter for UX111 (July 2025) — Detailed the facility readiness and data gap issues that triggered the CRL.
- Rocket Pharmaceuticals: FDA Acceptance of BLA Resubmission for Kresladi — Illustrates the impact of analytical uncertainty and the path to resolution.
- MeiraGTx 2025 Regulatory Update: Alignment on Expedited CMC PPQ for AAV-hAQP1 — A success story of utilizing RMAT designations for validation “right-sizing”.