Are you working on a new antibacterial or antifungal drug? If so, the Qualified Infectious Disease Product (QIDP) designation could be a game-changer in expediting its market approval, especially if your drug targets resistant infections. Part of the FDA’s Generating Antibiotic Incentives Now (GAIN) program, QIDP status offers substantial incentives for drugs that address serious or life-threatening infections caused by antimicrobial-resistant pathogens, a growing global health concern.
FDA’s QIDP Designation: The Game-Changer in Antimicrobial Drug Innovation
April 23, 2025
What is QIDP Designation?
The QIDP designation is granted by the FDA to an antibacterial or antifungal drug aimed at treating serious infections, particularly those caused by multidrug-resistant pathogens, including novel or emerging infectious pathogens, or to qualifying pathogens listed under Section 505E(f) of the 56 FD&C Act. This designation focuses on life-threatening infections, such as those caused by MRSA (Methicillin-resistant Staphylococcus aureus), vancomycin-resistant Enterococcus, Clostridium difficile, and other resistant bacteria. The goal is to fast-track the availability of critical treatments for infections that pose significant risks to patient health.
Pathogen List: Who’s in the Club?
One way for a drug to qualify for QIDP designation is that it must target infections caused by pathogens included on the FDA’s qualifying pathogens list. You can find it here: 21 CFR 317.2. The FDA’s pathogen list includes various foodborne organisms that frequently cause illness in the United States, including Clostridium botulinum, multidrug-resistant E. coli, and Salmonella.
The 5-Year Exclusivity: A Critical Advantage
The 5-year exclusivity extension (added to any exclusivity the marketing application receives upon approval) is one of the key benefits of QIDP status under the GAIN Act. This exclusivity is particularly valuable for maintaining commercial momentum, as it ensures a protected window for the drug’s market success. Note that this exclusivity applies only to human drugs submitted under section 505 of the Federal Food, Drug, and Cosmetic (FD&C) Act.
More Than Just QIDP: The Fast-Track Combo
- Fast Track Designation: This designation is granted to drugs addressing unmet medical needs or serious conditions, enabling frequent FDA interactions, rolling reviews, and potentially faster approval to help critical treatments reach the market sooner. Although QIDP designation makes a drug eligible for Fast Track, they are distinct processes. Fast Track must be specifically requested and granted separately.
- Priority Review: Drugs with this designation are expedited through the review process, typically taking 6 months instead of 10. It is granted to drugs offering significant improvements in treating serious conditions, allowing quicker availability to patients. The FDA gives priority review to the first application for a QIDP.
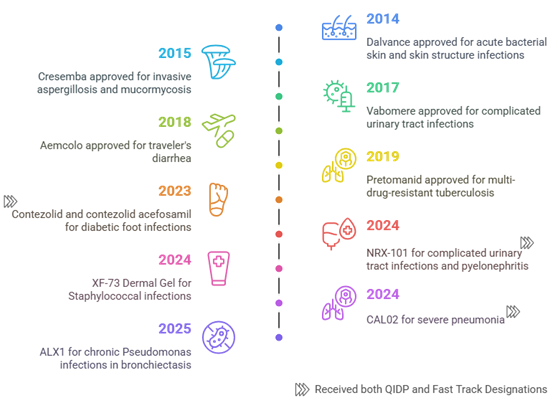
Examples of Drugs Granted QIDP Status (2013–2025)
Analysing FDA Approval Patterns for QIDPs
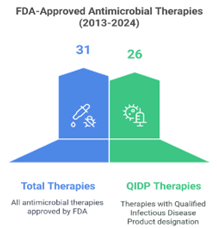
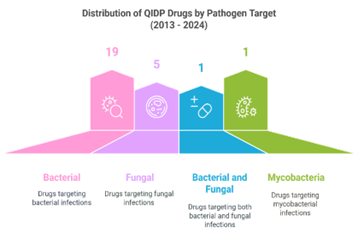
In 2023, three novel drugs—DefenCath (active against both bacteria and fungi), Rezzayo (active against fungi), and Xacduro (active against bacteria) and in 2024, Exblifep, Zevtera, and Orlynvah benefited from QIDP designation, priority review, and subsequent FDA approval. These drugs target both Gram-positive and Gram-negative bacteria. Beyond these approvals, several promising candidates—MET-X, XF-73, APC148-meropenem, NRX-101, CSA-131, and Cal02—have also attained QIDP status. However, these drugs remain in clinical trials and have yet to receive FDA approval for use.
The Request Process
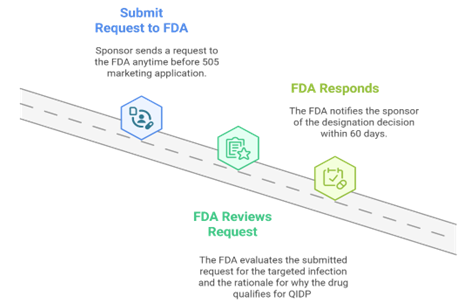
Wrapping Up: Why QIDP is a Game Changer
The QIDP designation is a powerful regulatory tool that can help expedite the development and approval of antibacterial and antifungal drugs aimed at resistant infections. With benefits like priority review, Fast Track status, and a 5-year exclusivity extension, QIDP offers a competitive edge for developers aiming to bring life-saving treatments to market faster. As the need for new antimicrobial treatments grows, QIDP provides an invaluable opportunity to make a significant impact on public health.
For the latest updates on regulatory news and industry best practices, subscribe to our newsletter and follow us on [Social Media Platform].
Disclaimer: This newsletter is for informational purposes only and does not constitute legal or regulatory advice. Please refer to the official FDA guidance document for detailed recommendations
References:
- S. FDA – Guidance for Industry – Qualified Infectious Disease Product Designation — Q&A, May 2021.
- Atillasoy, C., Elmansy, L., & Gourlias, P. (2023). A Decade in Review: An Analysis of the Qualified Infectious Disease Product (QIDP) Designation.Open Forum Infectious Diseases, 10 (Suppl 2), of ad500.2130.
- Compilation of CDER New Molecular Entity (NME) Drug and New Biologic Approvals for the year 2023
- Compilation of CDER New Molecular Entity (NME) Drug and New Biologic Approvals for the year 2024