In a world where rare diseases often go unnoticed, Japan has spent the last 25 years changing the narrative. Since the launch of its Orphan Drug Designation (ODD) program in 1993, Japan has become a global front-runner in delivering hope and treatments to patients living with rare conditions. With a patient-focused regulatory framework, strong government support, and increasing alignment with global standards, Japan’s ODD ecosystem has catalyzed the approval of hundreds of life-altering therapies, many of which reached patients faster than in other regions. This article explores how Japan’s proactive stance on rare disease drug development is shaping the future of global healthcare.
Transforming Treatment for the Few: Japan’s Legacy in Orphan Drug Development
May 14, 2025
The Human and Strategic Case for Japan’s ODD Program
Japan’s ODD program plays a vital role in improving the lives of patients with rare diseases. By providing financial and regulatory incentives, the program encourages the development of innovative treatments, offering hope to those who previously had limited options.
For companies developing orphan drugs, Japan’s ODD framework is essential for strategic regulatory planning. Understanding the differences in ODD criteria and incentives across major regulatory agencies, such as the FDA (U.S.), EMA (EU), TGA (Australia), CDSCO (India), South Korea (MFDS), and NMPA (China), can impact global regulatory strategies. This multi-regional approach will not only accelerate the development and approval of orphan drugs but also ensure broader patient access and sustained market exclusivity.
“In 2012, the EMA and PMDA launched a pilot collaboration on orphan medicines, aiming to foster mutual understanding and streamline global alignment in orphan drug designation.”
How Japan’s ODD Incentives Stack Up Worldwide
| Key Incentives | ||||||
|---|---|---|---|---|---|---|
| Region | Local Patient Population Prevalence | Grants/Subsidies for Clinical Research | Tax Credits for Clinical Testing | Fees Waiver for Marketing Applications | Priority Review of Marketing Applications | Market Exclusivity |
| Japan | <50,000 patients | ✓ | Up to 12% | ✓ | 10 years | |
| U.S. | <200,000 patients | ✓ | Up to 25% | ✓ | ✓ | 7 years |
| EU | <5 in 10,000 people | Varies by member states | ✓* | ✓ | 10 years | |
| Australia | <5 in 10,000 people# | ✓ | ✓ | 5 years | ||
| South Korea | <20,000 patients | Up to 20% | 50 – 75% reduction | ✓ | 6 years | |
| India | <5,00,000 patients | Up to 25% | ✓ | ✓ | 7 years | |
| China | <1 in 10,000 people | ✓ | ✓ | 6 years | ||
Milestones and Momentum in Japan’s ODD Journey
- PMDA has seen a steady increase in designations. Between 2019 and 2018, 432 orphan drugs were designated, with a high marketing approval rate of 75%.
- Strong government support for rare disease drug development through funding and regulatory incentives.
- A notable shift toward biologics and gene therapies in recent years.
Outpacing Global Averages in ODD Outcomes:
- Higher Approval Proportion: Japan’s approval rate is higher compared to the U.S. (17%) and EU (8%), due to emphasis on feasibility during designation.
- Disease Prevalence: Most approvals for conditions affecting 1,000 to 10,000 patients, followed by those with fewer than 500 patients.
- Focus Areas: Oncology and central/peripheral nervous system disorders have the highest proportion of ODD designations.
Innovative Approaches:
- Early Adoption: Japan has approved therapies like edaravone for ALS and nivolumab for melanoma ahead of other regions.
- SAKIGAKE Designation: Introduced in 2015, provides an accelerated regulatory pathway for innovative orphan drugs, similar to the U.S. Breakthrough Therapy and EU PRIME programs.
Future Outlook:
- Globalized Clinical Trials: There is a move towards globalized clinical trials and patient registry initiatives to support regulatory decisions. Nusinersen for spinal muscular atrophy is an example of multi-regional trial success. Patisiran, Canakinumab, Mepolizumab – show global cooperation in rare disease drug development.
- Real-World Evidence: Increased use of real-world evidence to support orphan drug development. Sebetralstat designation in 2025 for the treatment of Hereditary Angioedema (HAE) was supported by data from international clinical trials and patient-reported outcomes.
Examples of ODD Approvals
| Drug | Indication | Approval |
|---|---|---|
| Lumasiran | Treatment of primary hyperoxaluria type 1 (PH1) | Apr 2024 |
| Vutrisiran | Treatment of hereditary transthyretin-mediated amyloidosis (hATTR) | Jun 2024 |
| Tezepelumab | Treatment of severe asthma | Aug 2024 |
| Odevixibat | Treatment of progressive familial intrahepatic cholestasis (PFIC) | Oct 2024 |
| Maralixibat | Treatment of Alagille syndrome | Dec 2024 |
| Akuugo | Treatment of Duchenne muscular dystrophy (DMD) | Apr 2025 |
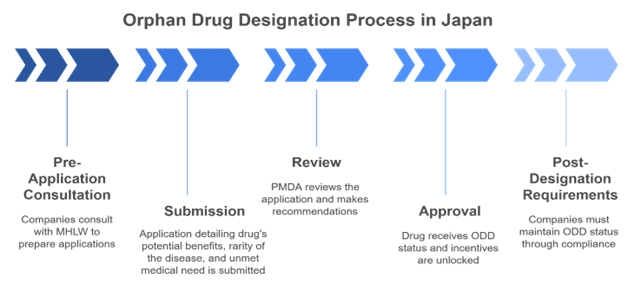
The Bottom Line
Japan’s legacy in orphan drug development is more than a regulatory success story—it’s a testament to what’s possible when innovation, policy, and compassion converge. As the country continues to embrace global collaboration, real-world evidence, and next-generation therapeutics, its ODD framework is well-positioned to lead the next era of rare disease breakthroughs. For pharma innovators worldwide, Japan isn’t just a market—it’s a strategic launchpad for impact, inclusion, and progress of rare diseases.