Registration & Listing of Cosmetic Facilities and Products in the US
September 17, 2025
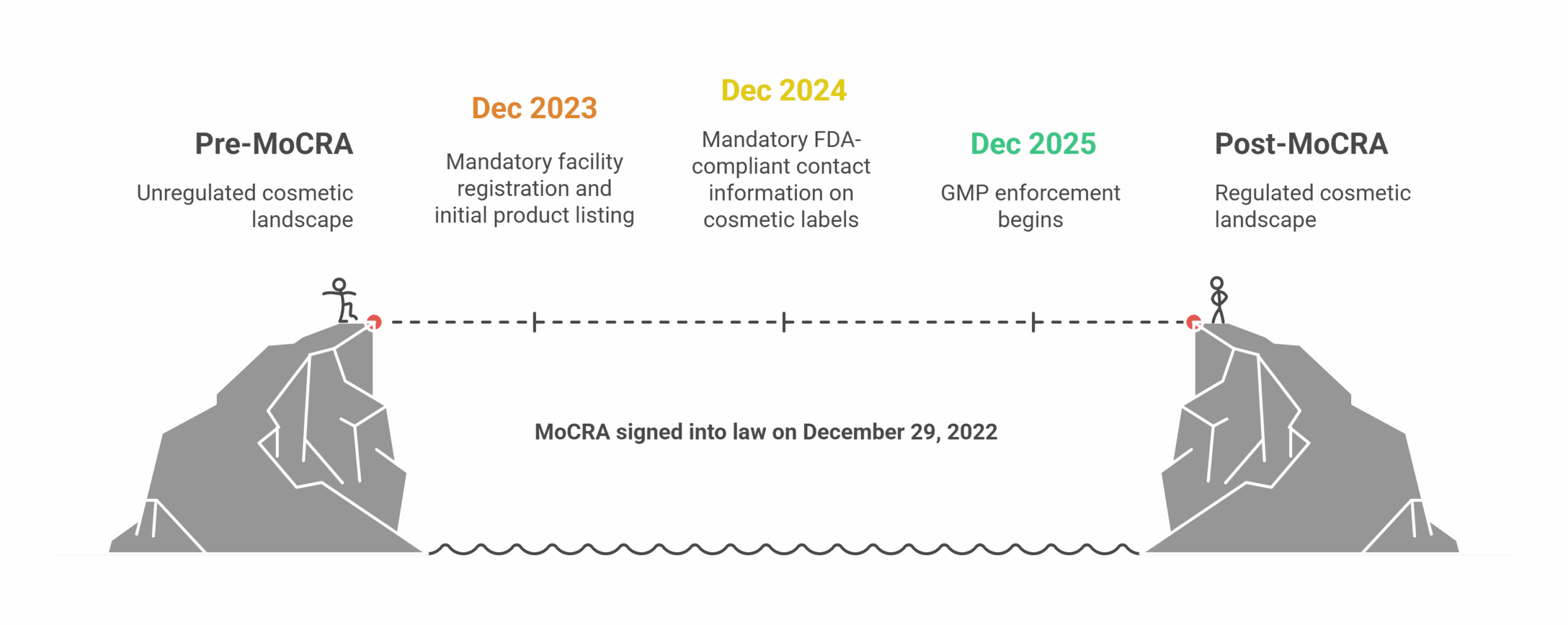
2025 marks a regulatory turning point with the FDA’s full enforcement of the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), transforming the landscape for cosmetic manufacturers, importers, and contract partners. It is the most significant update to the U.S. cosmetics regulatory framework in over 80 years. Facility registration and product listing are no longer voluntary, and the FDA’s enhanced oversight brings both new opportunities and risks for market leaders.
This newsletter details the MoCRA evolution, key FDA obligations, enforcement trends, global comparisons, and practical compliance strategies to help industry professionals navigate this rapidly changing landscape in 2025 and beyond.
Cosmetic Regulatory Milestones in the US
Key Intelligence
- MoCRA significantly expands the FDA’s authority, allowing suspension of facility registrations if public health risks are found—a first in U.S. cosmetics regulation history.
- Adverse event reporting now requires brands to notify the FDA of serious health-related complaints within 15 business days, accelerating the FDA’s safety response.
- The Act closes regulatory gaps, aligning cosmetic oversight closer to that of foods, drugs, and medical devices, establishing a new standard of accountability.
- Foreign companies must designate and maintain active U.S. Agents responsible for FDA communications, compliance records, and adverse event coordination.
- The FDA’s expanded database provides public access to cosmetic product and facility data, increasing transparency but also reputational exposure for non-compliant companies.
Case Study: Rising FDA Enforcement Highlights MoCRA Compliance Gaps
• A recent analysis from Registrar Corp in November 2024 revealed that 48% of imported cosmetics sold at America’s top 25 retailers were not compliant with MoCRA registration and listing requirements.
• FDA logged 5,000+ consumer health complaints in 2023, including severe reactions and permanent injuries.
• This non-compliance spanned over 135,000 products, posing consumer safety risks, including severe allergic reactions and permanent injuries reported to the FDA.
• In response, the FDA has aggressively ramped up enforcement actions, including warnings, product recalls, and requests for rapid corrective measures.
FDA Regulatory Requirements
|
Requirement |
Who Must Comply |
What’s Required |
Frequency |
|
Facility Registration |
Domestic and foreign manufacturers/processors |
FDA Establishment Identifier (FEI) + FDA Form 5066 via Cosmetics Direct |
Every 2 years |
|
Product Listing |
Responsible Person (named on label) |
FDA Form 5067 + ingredient details |
Annual + upon changes |
|
Foreign Facilities |
Must appoint a U.S. Agent |
Serve as FDA contact |
Continuous |
|
Exemptions |
Some small businesses, drug/device overlaps |
Documented exemptions |
Reviewed annually |
For a detailed guide on obtaining and understanding the importance of the FEI number, see our article: How to Apply for Your FDA FEI Number?

“The agency will rely on registration and listing to identify risky products, facilitate recalls, plan inspections, and enforce compliance.”Linda Katz, M.D., M.P.H., Director, FDA Office of Cosmetics & Colors
2025 Enforcement Snapshot:
As of January 1, 2025, the U.S. FDA reported a nearly 20-fold increase from the pre-MoCRA Voluntary Cosmetic Registration Program (VCRP) totals of 5,176 facilities and 35,102 products:
• 9,528 active cosmetic product facility registrations, including both domestic and foreign manufacturing sites.
• 589,762 unique cosmetic product listings registered under the mandatory MoCRA requirements
• Foreign facilities constitute a significant share, with China accounting for about 44.7% of registered establishments.
Strategic Global Comparison
|
Country |
Jurisdiction |
Facility Registration |
Product Listing |
Responsible Person |
Labeling |
Exemptions |
|
|
U.S. Food and Drug Administration (FDA) |
Mandatory biennial |
Annual full ingredient listing |
US Responsible Person required |
Contact info required on label |
Small business exemptions apply |
|
|
European Medicines Agency (EMA) / European Commission |
No; Responsible Person (RP) |
Notification via Cosmetic Product Notification Portal (CPNP) |
RP required |
RP address and batch code |
Limited; mostly R&D related |
|
|
Medicines and Healthcare products Regulatory Agency (MHRA) |
No; RP required |
Notification via UK Submit Cosmetic Product Notification (SCPN) Portal |
RP required |
RP address and batch code |
Similar to the EU |
|
|
Health Canada (HC) |
No; cosmetic notification form |
Notification within 10 days of sale |
Manufacturer or importer |
Label contact is often required |
Few, varies by province |
|
|
National Medical Products Administration (NMPA) |
Registration or filing varies |
Registration or filing required |
Chinese RP |
Local labeling, testing required |
None; strict enforcement |
Why This Matters Beyond Compliance
From a marketing and commercial perspective, facility registration and product listing are reshaping how brands operate:
- Supply chain leverage: Retailers and distributors are increasingly asking for proof of listing as part of vendor qualification.
- Consumer trust: In an era of transparency, being able to say “Our products are fully FDA-listed” is becoming a brand value proposition.
- Operational discipline: Companies that build robust change-control processes are discovering fewer product launch delays.
Closing Thoughts
As MoCRA continues to reshape the cosmetics landscape, expect the FDA to step up its oversight with smarter tools and data-driven enforcement. This means greater transparency and a closer look at product safety, labeling, and supply chains. For brands, staying ahead means building strong compliance programs, keeping U.S. Agent relationships active, and preparing for evolving requirements—both at home and globally. Embracing these changes isn’t just about meeting regulations; it’s about gaining trust, reducing surprises, and positioning your brand for long-term success in a rapidly changing market.
References
- FDA Registration & Listing of Cosmetic Product Facilities and Products
- Modernization of Cosmetics Regulation Act of 2022 (MoCRA)
- FDA Guidance for Industry: Registration and Listing
- Registrar Corp: MoCRA Compliance Guide
- Elchemy Regulatory Practice: Cosmetic Industry Regulations
- Capote Law Firm: FDA Cosmetic Updates

