PreCheck Program: FDA’s Roadmap for US Drug Manufacturing
February 12, 2026
After 30 years of prioritizing cost-savings through offshoring, the U.S. pharmaceutical supply chain is dangerously lean. The nation now relies on overseas facilities for 53% of brand-name drugs and 69% of generics, with only 9% of registered API sites located domestically, while India and China account for 44% and 22%, respectively. On February 1, 2026, the FDA launched the PreCheck Program, a new regulatory fast‑pass designed to strengthen domestic manufacturing, reduce CMC risk, and front-load facility readiness assessments in alignment with Executive Order 14293. Unlike EMA or PMDA, the FDA is leading this effort globally, with no comparable programs available internationally.
This newsletter edition gives domestic sponsors and manufacturers a clear roadmap of how to prepare strong PreCheck submissions and what the FDA will prioritize in the 2026 selection cycle. It also outlines the practical steps facilities can take now to demonstrate readiness, innovation, and alignment with U.S. onshoring goals.
“After 35 years of globalists taking pharmaceutical manufacturing overseas, the FDA is taking bold steps to bring it back. The PreCheck program is one of several powerful incentives we are providing to make the U.S. pharmaceutical manufacturing sector more resilient and competitive.”– FDA Commissioner Marty Makary, M.D., M.P.H. (February 1, 2026)
The PreCheck Engine: A Two-Phase Strategic Advantage
The PreCheck program operates on a structured, two-phase framework designed to de-risk the most volatile variable in drug development: facility readiness. This shift allows the FDA to evaluate the “bricks and mortar” independently of the “molecule,” providing a level of procedural predictability previously unseen in CMC reviews.
Phase 1: The Facility Readiness Phase
Purpose: Reduce risk of late-stage CMC or facility-related FDA rejections.
Key elements:
-
- Early, frequent FDA engagement during design, construction, and pre-production.
- Submission of Type V Drug Master Files (DMFs) providing:
- Facility layout
- Quality management systems
- Pharmaceutical Quality System elements
- Quality Maturity practices
- DMF becomes referenceable in later product applications, giving sponsors early assurance of facility acceptability.
Benefits:
-
- Avoids costly design flaws uncovered only during pre-approval inspections.
- Helps CDMOs overcome first-mover disadvantage by demonstrating readiness to clients.
Phase 2: The Application Submission Phase
Purpose: Focuses on streamlining the NDA, ANDA, or BLA CMC review process.
Key elements:
-
- Pre-application meetings with FDA to align on CMC expectations.
- Early FDA feedback on application structure and quality data.
- Reliance on earlier Type V DMF information to avoid duplicative facility assessments.
Outcome:
The ultimate benefit is a streamlined Pre-Approval Inspection (PAI). The PAI becomes a targeted exercise focusing on product-specific risks rather than baseline facility compliance, significantly reducing the risk of a high-stakes delay at the finish line.
Eligibility: The Seven-Seat Cohort
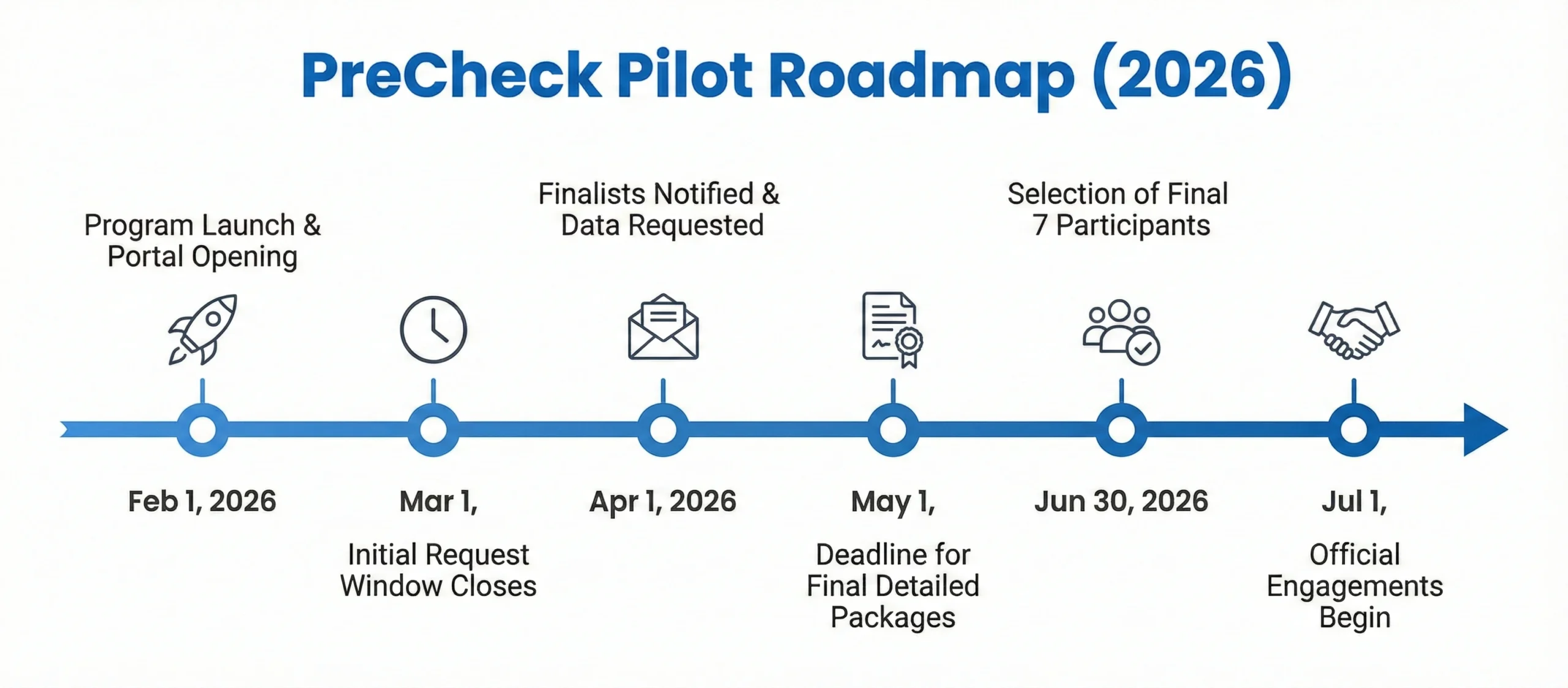
The FDA has limited the 2026 cohort to just seven facilities, making selection highly competitive. To qualify, a facility must be a new domestic site. Extensions or expansions of existing sites are currently ineligible.
Selection Priorities
- Product Criticality: Priority goes to sterile injectables, large volume parenterals, and drugs on the FDA shortage list.
- Domestic Sourcing: Facilities using U.S.-sourced APIs and key starting materials are favored.
- Innovation: Sites utilizing modular construction, advanced automation, or continuous manufacturing receive higher consideration.
- Expeditious Timeline: Applicants must quantify how PreCheck will minimize their specific time-to-market.
- Company Qualifications: The program seeks companies with proven pharmaceutical manufacturing experience, whether through existing commercial operations, strategic partnerships, or a robust plan to build in-house expertise.
Note: Sponsors must also commit to submission of an NDA, ANDA, or BLA during the pilot and to actively manufacture at the site for at least three years post-approval.
Stakeholder Feedback and Industry Sentiment
Industry sentiment regarding the PreCheck rollout has been strongly positive, with requests for:
-
- Early Engagement Strategy: Stakeholders have expressed a high demand for early, durable engagement throughout all stages of facility development over formal end-of-stage reviews.
- DMF Content Clarity: There is a significant call for clear, standardized expectations for Type V DMF content. Industry groups have requested a defined boundary between reusable DMF data and product-specific Module 3 content to avoid administrative redundancy.
- CDMO Inclusion: Enhanced support for Contract Development and Manufacturing Organizations (CDMOs) has been a primary feedback pillar. Manufacturers have requested clarification on how CDMO-owned facilities can reference a single PreCheck status across multiple sponsor applications.
The FDA has acknowledged this feedback and is currently revising program details, although many remain pending until the final Phase 1 selection notifications in April 2026.
Operational Considerations for Applicants
- Tight Documentation Window: The short preparation window (February 1 – March 1) poses significant challenges for firms that have not yet formalized their Quality Management System (QMS) descriptions or facility layouts into an eCTD-ready format.
- Undefined Scoring Weight: As of February 2026, the specific weighting of the selection criteria, such as how much Innovation offsets a slightly longer Timeline, remains internal to the Agency.
- Competitive Pressure: With only seven seats available, the hurdle for demonstrating alignment with national priorities is exceptionally high.
Strategic Steps for Companies Preparing for PreCheck Pilot Program
To secure a position in the 2026 cohort or prepare for future expansions of the program, companies should adopt the following strategic posture:
-
- Finalize Core Documentation: Immediately gather all facility design documents, PQS descriptions, and readiness milestones to meet the March 1 deadline.
- Draft a Robust Type V DMF: Ensure your dossier includes detailed facility layouts, equipment strategies, and Quality Management Maturity (QMM) assessments.
- Establish a Cross-Functional Team: Create an internal PreCheck Readiness Team that bridges Quality Assurance, Regulatory Affairs, Engineering, and Validation.
- Prioritize Supply Chain Resilience: Quantify exactly how your facility will contribute to U.S. supply chain resilience, focusing on domestic sourcing of key starting materials (KSMs).
- Sponsor-CDMO Alignment: If you are a CDMO, engage your potential product sponsors early. Their commitment to submit product applications (NDAs/BLAs) from your site is a baseline requirement for eligibility
Sponsor Pro-Tip: Do not view PreCheck in isolation. Combine it with other incentives like the Commissioner’s National Priority Voucher (CNPV), which can shorten review times for onshore products to just 1–2 months.
How Can BLA Regulatory Help?
Navigating the high-stakes FDA PreCheck Program requires a delicate balance of strategic CMC planning and proactive Agency engagement. BLA Regulatory helps sponsors mitigate the primary drivers of non-approval, such as CMC deficiencies (responsible for 20% of refusals) and facility-related Complete Response Letters (CRLs), by functioning as a seamless extension of your internal team.
Our highly experienced consultants, including FDA veterans, provide the specialized expertise needed for therapeutic proteins and monoclonal antibodies to ask the right questions and present data effectively during pre-operational reviews. We lead your Regulatory Gap Analysis to assess alignment with national priorities, develop robust Type V DMFs that withstand Phase 1 scrutiny, and conduct Mock FDA Inspections to identify PAI risks before regulators arrive. As your U.S. FDA Agent, we streamline your path to approval by crafting “Established Conditions” under ICH Q12, ensuring your domestic facility remains both agile and compliant from groundbreaking to commercial launch.
References
- (https://www.fda.gov/drugs/news-events-human-drugs/fda-public-meeting-onshoring-manufacturing-drugs-and-biological-products-09302025)
- (https://www.fda.gov/news-events/press-announcements/fda-launches-precheck-pilot-program-strengthen-domestic-pharmaceutical-manufacturing)
- (https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-precheck-implementation-roadmap)
- (https://www.fda.gov/industry/fda-manufacturing-precheck-pilot-program)
- Commissioner’s National Priority Voucher (CNPV) Pilot Program: Accelerating Public Health Goals
- (https://www.fda.gov/media/171705/download)
- (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ich-q12-implementation-considerations-fda-regulated-products)
- (https://www.federalregister.gov/documents/2025/01/17/2025-01182/use-of-a-type-v-drug-master-file-for-model-master-file-submissions-to-support-abbreviated-new-drug)
- (https://www.fda.gov/about-fda/fda-commissioner/fda-accomplishments-american-people)
- (https://www.reedsmith.com/articles/fda-inspections-in-2025-heightened-rigor-data-driven-targeting-and-increased-surveillance/)
- (https://redica.com/fda-increased-for-cause-inspections-almost-250-in-2025/)
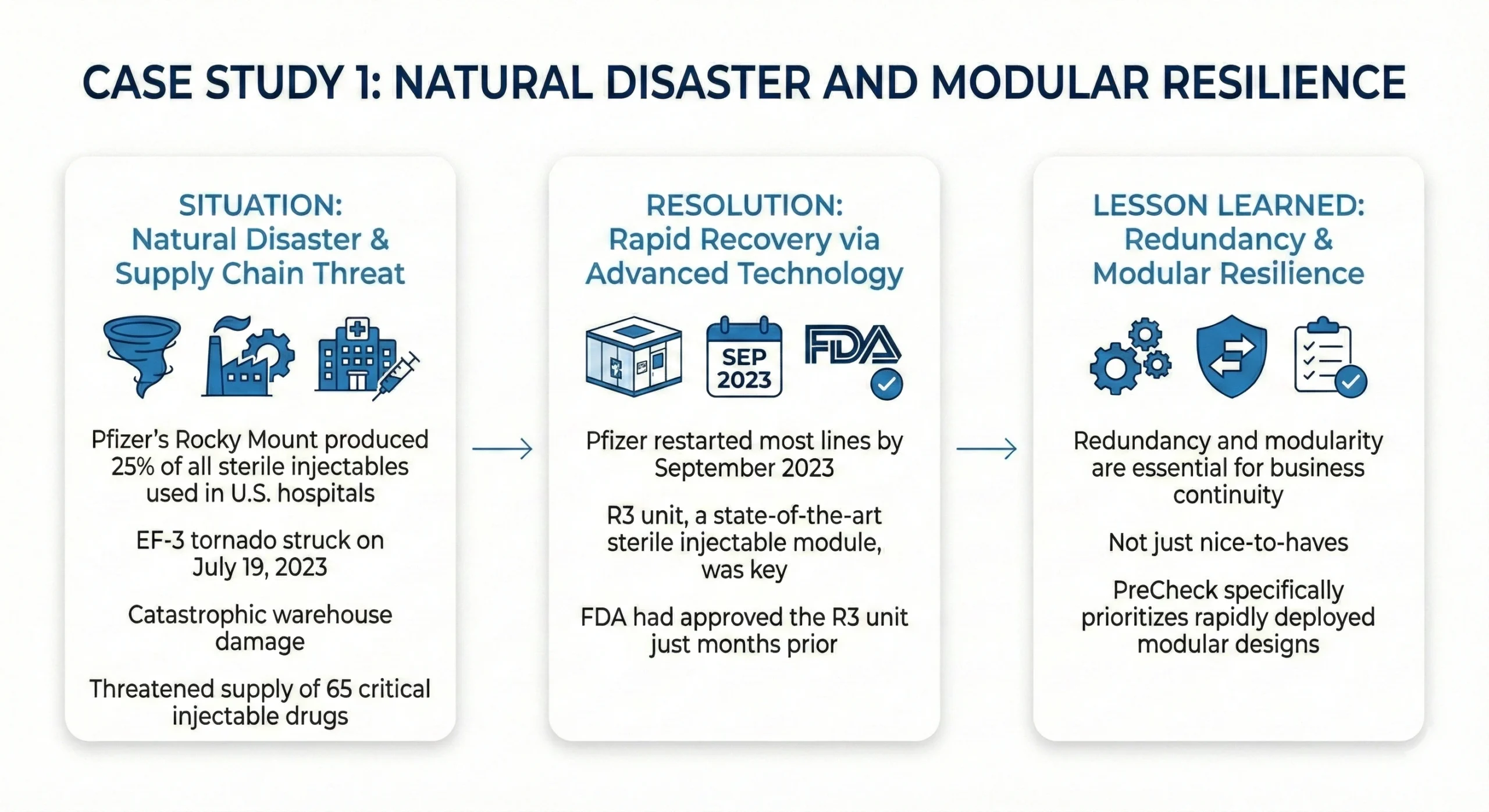
- (https://insights.pfizer.com/rocky-mount)
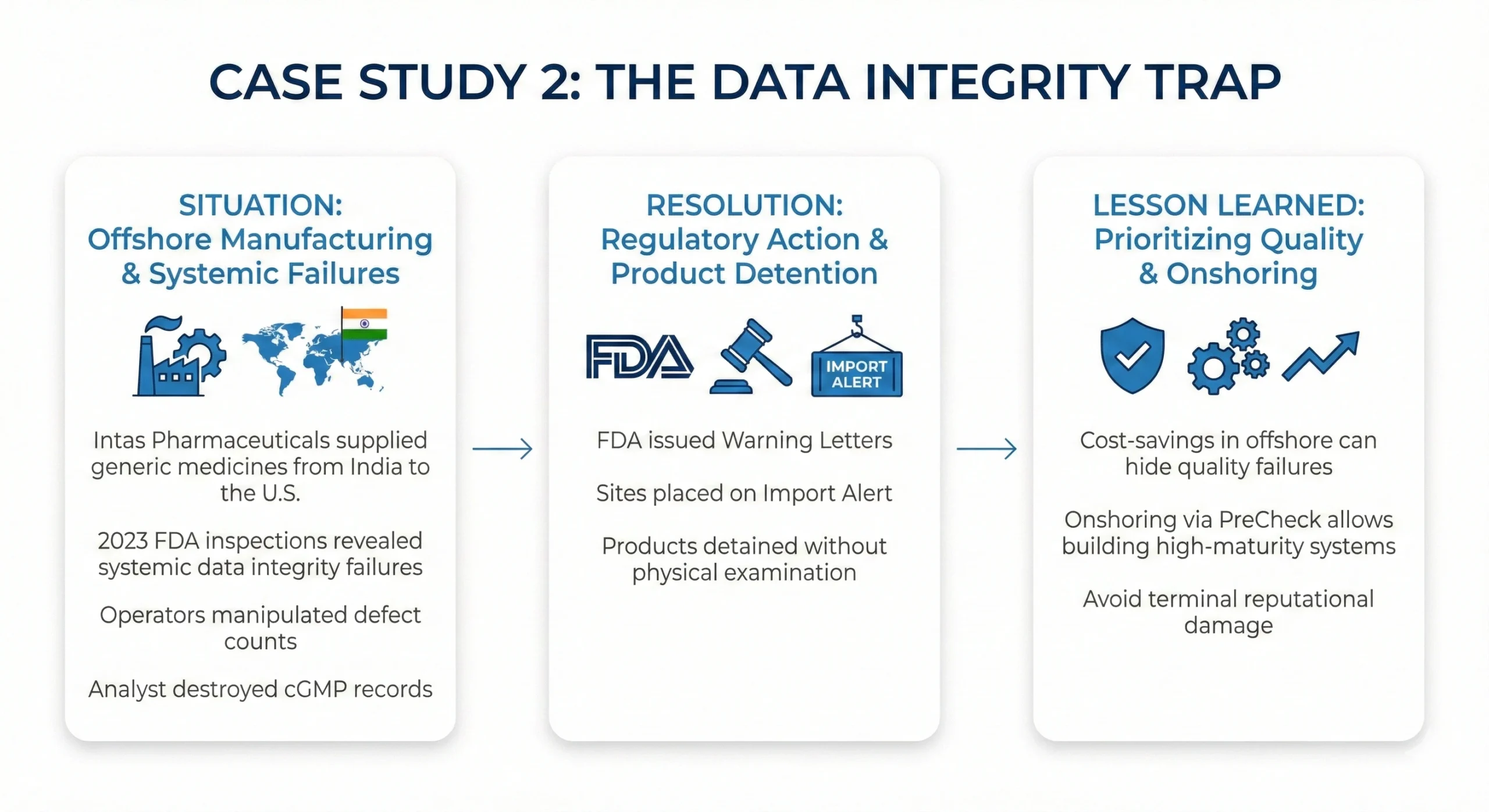
- (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/intas-pharmaceuticals-limited-662868-11212023)
- (https://www.pharmaceutical-technology.com/comment/fda-inspection-reveals-critical-supply-chain-vulnerabilities/)
- (https://www.fda.gov/drugs/drug-master-files-dmfs/types-drug-master-files-dmfs)
- (https://bla-regulatory.com/services/)
- (https://www.federalregister.gov/documents/2025/05/05/2025-14293/regulatory-relief-to-promote-domestic-production-of-critical-medicines)