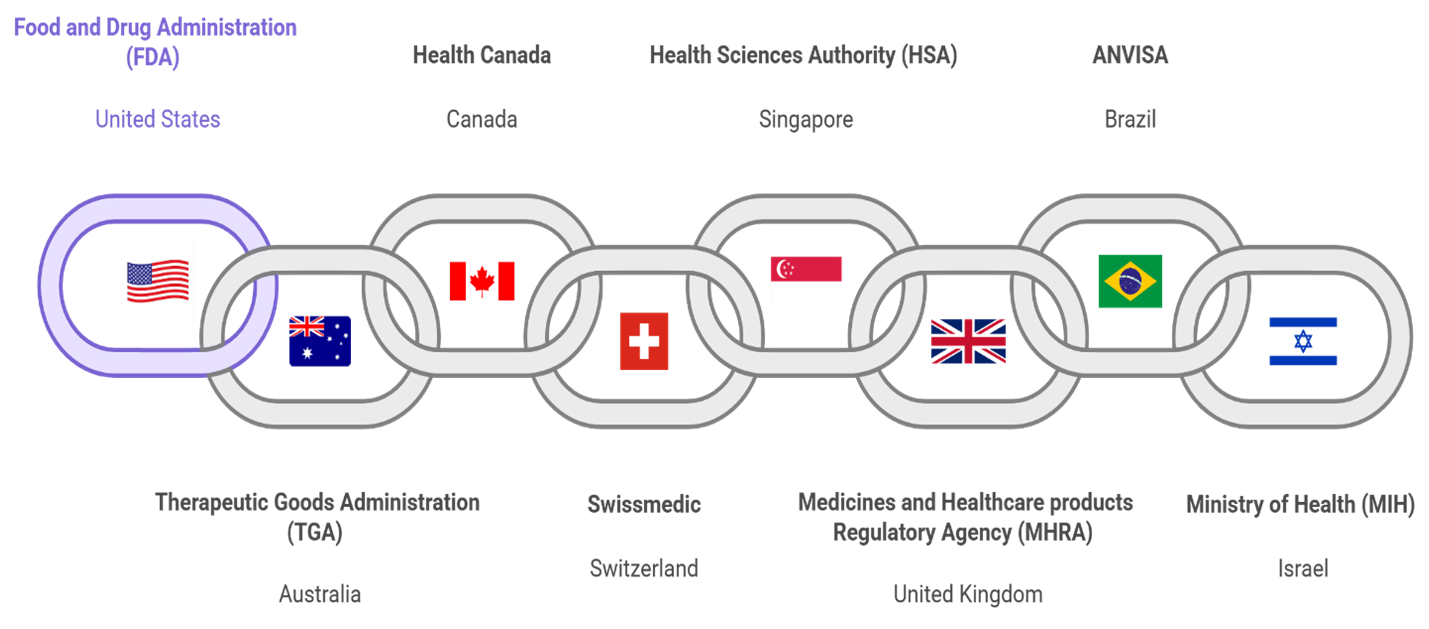
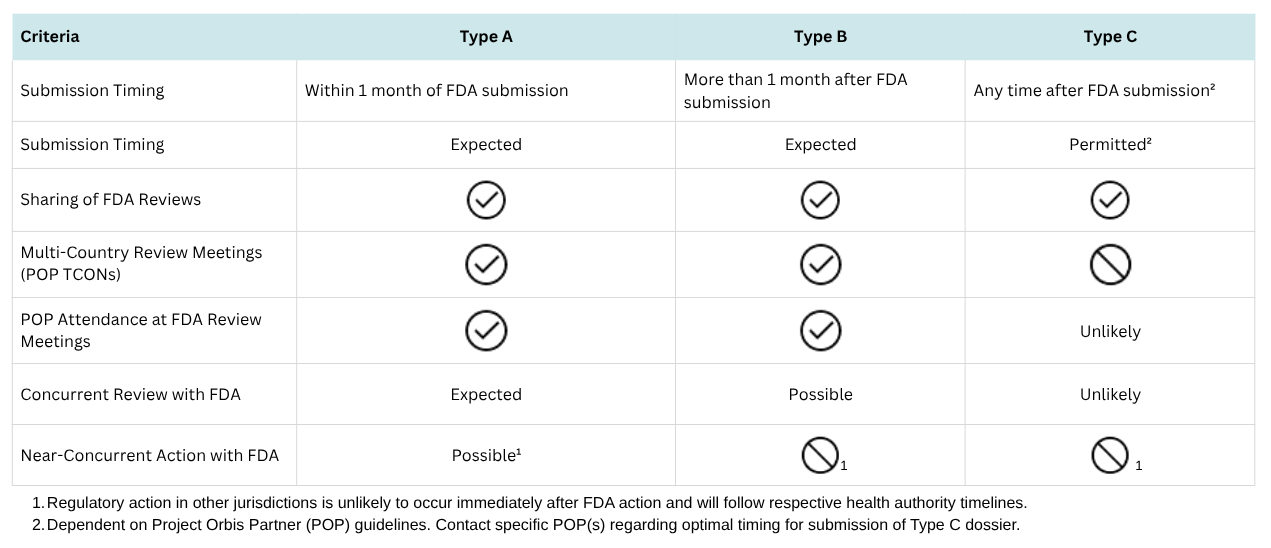
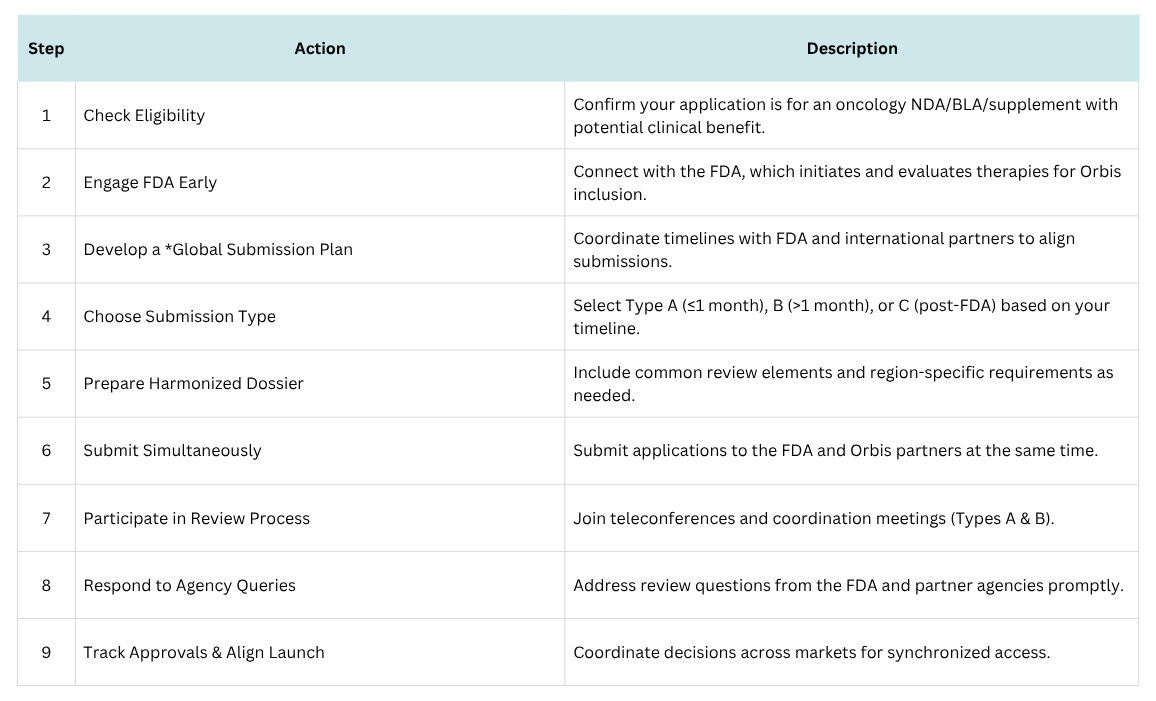
Launched by the FDA’s Oncology Center of Excellence in 2019, Project Orbis is an international collaboration framework that allows for the concurrent submission and review of oncology products among participating regulatory agencies. This initiative aims to expedite patient access to promising cancer therapies by facilitating simultaneous regulatory reviews and approvals worldwide.
Since 2019, multiple life-saving therapies have received approval, with more on the horizon. This newsletter explores Project Orbis’s mission, recent developments, future opportunities, and guidance for companies seeking to participate.