Clinical trial disclosure compliance is no longer just about good documentation – it’s about timing, accuracy, and transparency across borders.
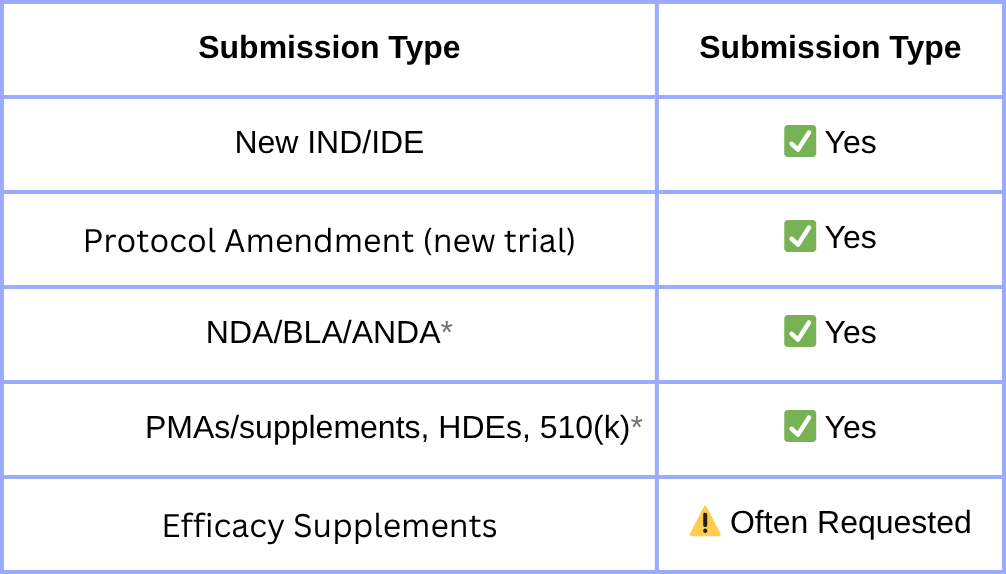
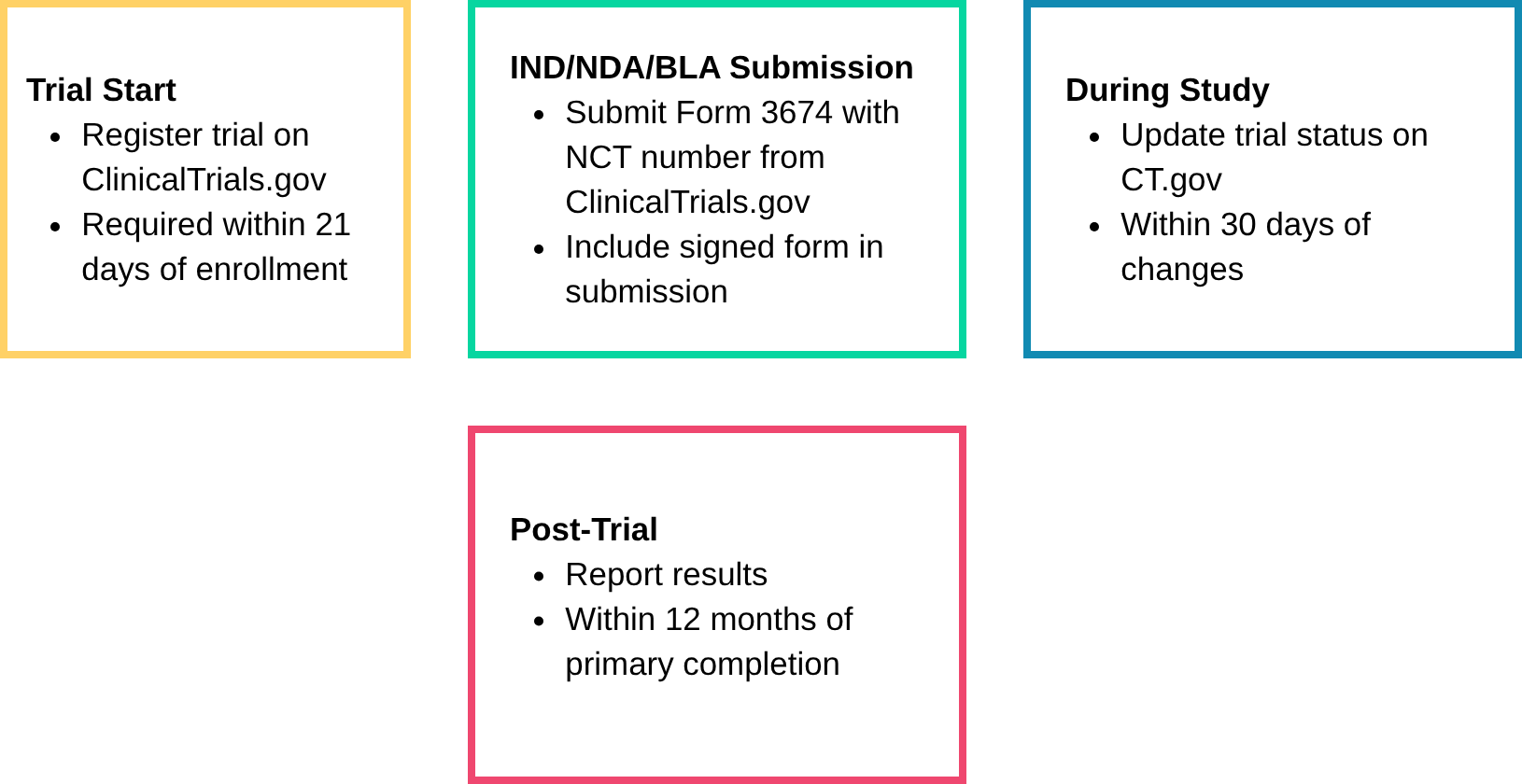
FDA Form 3674 is a required certification for nearly all U.S. clinical submissions, confirming trial registration and results reporting on ClinicalTrials.gov under the FDA Amendments Act (FDAAA) of 2007. But many teams still get it wrong, and the consequences include Refuse-to-File letters, submission delays, and audit findings. And now it’s not just the FDA watching. Global regulators—the EU, UK, Canada, and Australia—are tightening their own disclosure rules.
This newsletter breaks down:
- When Form 3674 is required (and often overlooked)
- Global equivalents and disclosure timelines
- Common missteps that delay approvals
- What top-performing clinical and regulatory teams do to stay ahead
🔍 Common Misunderstandings
Scenario: A cardiovascular trial was registered early, and results were posted promptly on clinicaltrials.gov.
Outcome: The transparency led to increased exposure, higher patient enrollment in follow-up studies, and positive feedback from advocacy groups.
Lesson: Compliance isn’t just about avoiding penalties—it builds credibility.
⏱️ Clinical Trial Disclosure Compliance Timeline (U.S.)
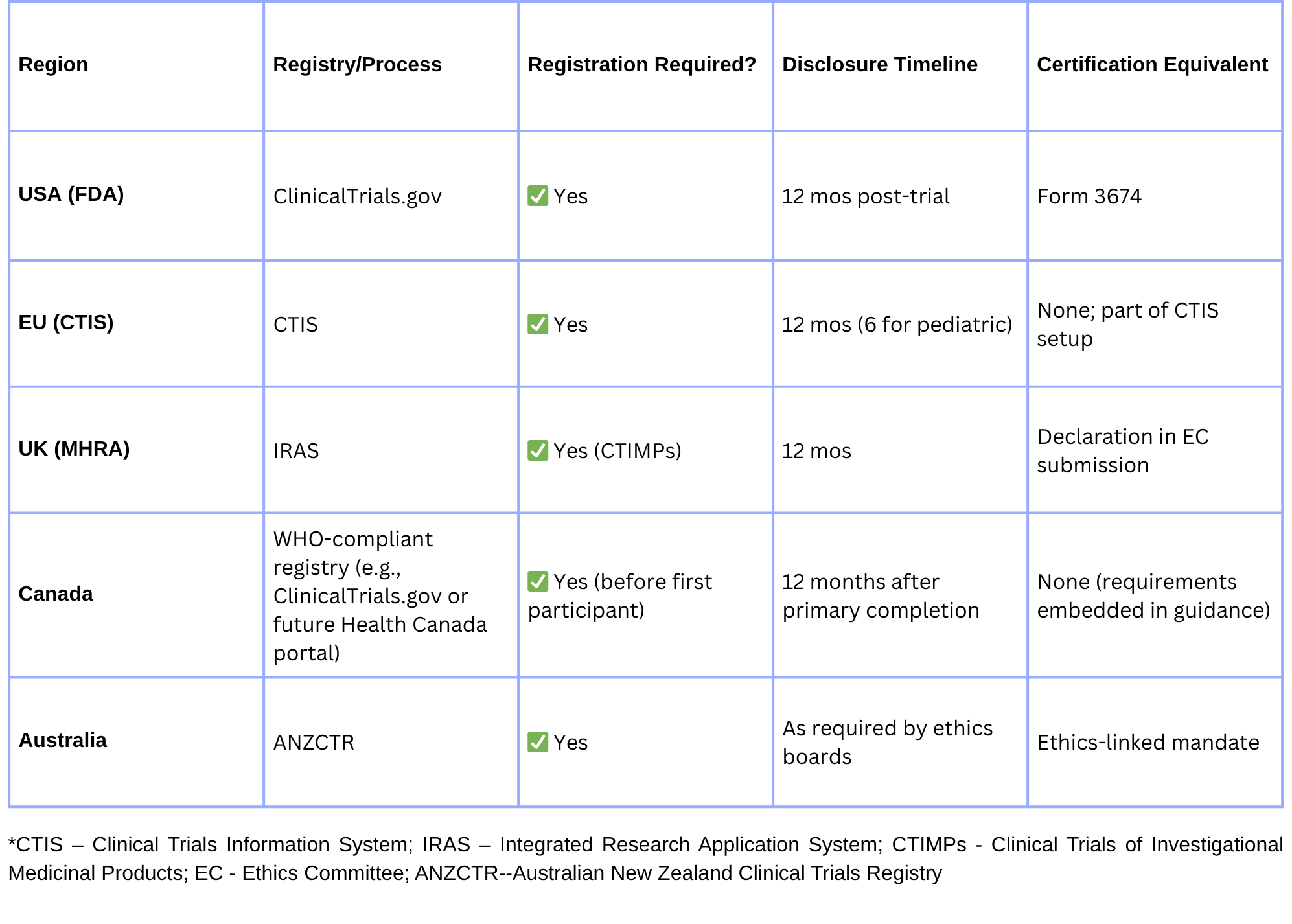
🌍 How Other Regulators Address Trial Disclosure
Harmonize registry entries across CT.gov, CTIS, and ANZCTR to avoid mismatched trial metadata.
Here’s where most people run into challenges with this form:
- ✅ Determining whether the clinical trial is “applicable”
Not every study qualifies as an “applicable clinical trial” (ACT). Figuring out if a trial meets the ACT criteria can be tricky — it depends on the type of product, the phase, whether it has U.S. sites, and if it falls under FDA jurisdiction. Many sponsors struggle to correctly interpret the FDA and NIH definitions.
- ✅ Matching the submission timelines
FDA requires you to certify at the time of NDA, BLA, IND, IDE, PMA, or 510(k) submissions. But ClinicalTrials.gov has its submission deadlines (see above). Ensuring you’re aligned on both sides is a compliance headache.
- ✅ Accurate and complete reporting
Even if you certify on the form, if the actual records on ClinicalTrials.gov are missing or incomplete, you can still face FDA or NIH enforcement. So, it’s not just filing the form — it’s about ensuring the underlying data is right.
- ✅ Understanding the penalties
Some companies underestimate the risks. Certifying compliance when you’re not compliant can lead to civil monetary penalties or other regulatory action.
- ✅ Coordination across teams
The regulatory team submitting the FDA application may not be the same team handling ClinicalTrials.gov reporting. Poor internal coordination can lead to gaps or mistakes.
The FDA is considering harmonization with EU CTIS data requirements post-2025, which could introduce multi-region certification checks.
🛠️What You Can Do: Best Practices for Global Sponsors, Regulatory, & Clinical Teams
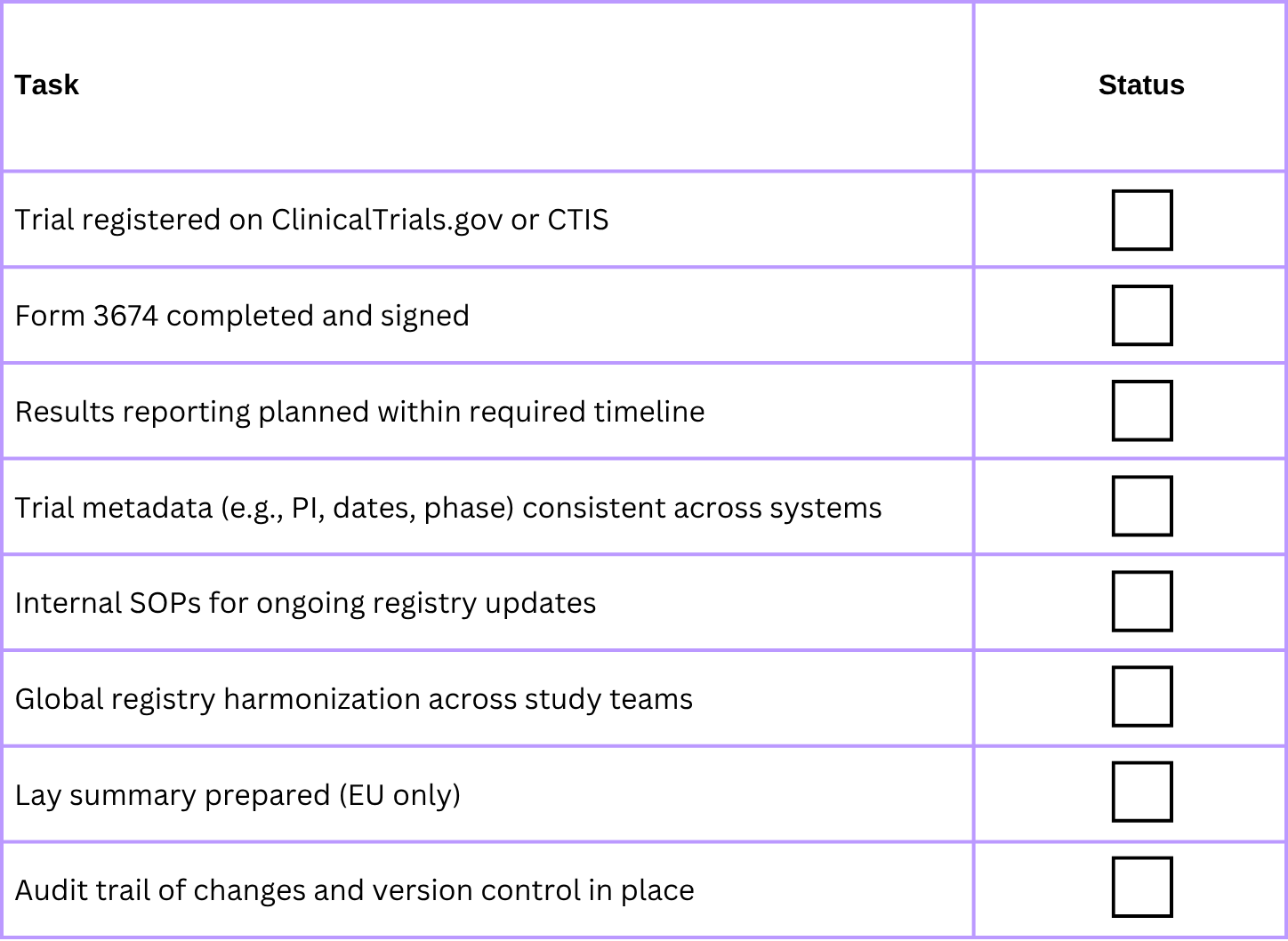
✅ Sponsor Readiness Checklist
Conclusion: Compliance as a Strategic Advantage
In a landscape where trial transparency is increasingly linked to public accountability, early and consistent registration practices give sponsors a distinct advantage. It ensures regulatory momentum, avoids costly surprises, and positions your organization as a credible, ethical leader in clinical research.
And with global regulators moving toward harmonization, it’s never been more important to think beyond the U.S. Form 3674 and build a global clinical trial disclosure strategy that supports your submissions across borders.
📩 Need help with Form 3674 or global registry strategy?
Let’s streamline your process.
🔗 Ask for:
- Form 3674 sample package
- Global trial registration tracker (Excel template)
- Regulatory compliance calendar (customized to your pipeline)