For decades, the dialogue between the FDA and drug sponsors was the industry’s best-kept secret. If an application didn’t cross the finish line, the “why” was often buried in a Complete Response Letter (CRL) that only the sponsor ever saw. That era is officially over.
Under the leadership of Commissioner Dr. Marty Makary, the FDA has traded its “black-box culture” for a policy of “Radical Transparency.” Following a preliminary release of 200 historically approved application letters in July 2025, the Agency announced on September 4, 2025, that it will now publish CRLs in real-time for all New Drug Applications (NDAs) and Biologics License Applications (BLAs), regardless of approval status.
For sponsors, this means that the specific scientific, clinical, and manufacturing deficiencies cited by the FDA will soon be public record shortly after issuance. At BLA Regulatory, LLC, we believe this shift demands a proactive transparency-first regulatory strategy.
The Disclosure Gap: Why the FDA is Moving Fast
The sudden pivot toward transparency was driven by a massive information asymmetry in the market. A landmark study published in the British Medical Journal (BMJ) served as the primary catalyst: researchers found that when sponsors announced a non-approval, they omitted approximately 86% of the safety and efficacy concerns cited by the FDA. Furthermore, roughly 41% of the time, sponsors failed to mention that the FDA had requested entirely new clinical trials.
Commissioner Makary’s stance is clear: “Although the company owns the proprietary information… they don’t own the thinking of the FDA reviewers. That’s the public domain.”
2025: The Year the Curtain Rose
The transition happened in two major waves that reshaped the regulatory landscape:
- July 2025: The FDA released over 200 historical CRLs for products that were eventually approved. While many were already tucked away in post-approval packages, centralizing them in the openFDA database made it easier than ever to spot trends and conduct peer benchmarking.
- September 2025: The “Radical” pivot. The agency released 89 CRLs for applications that were currently pending, withdrawn, or abandoned. This marked the first time the public gained a front-row seat to products that never made it to market. Moving forward, newly issued CRLs will be released promptly after issuance.
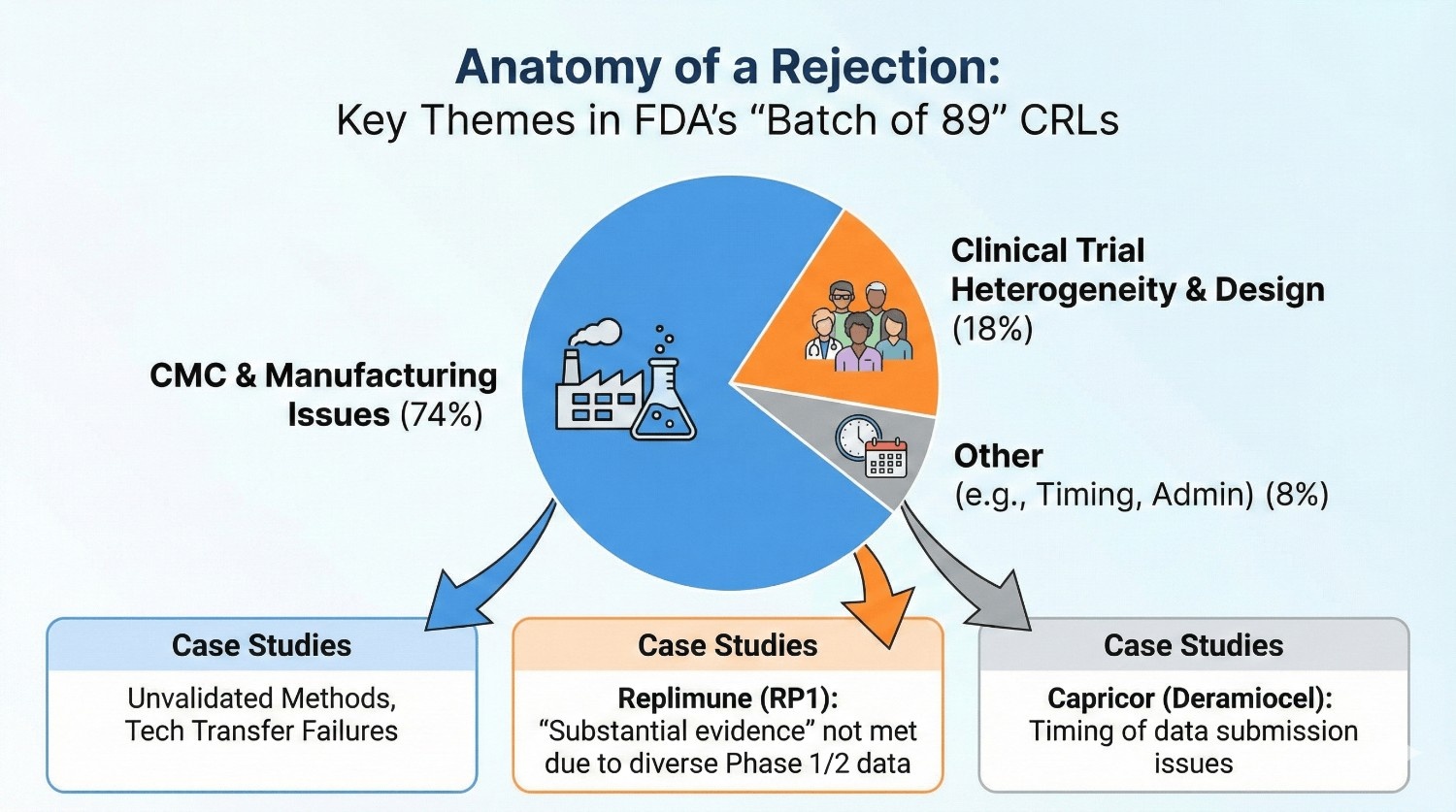
The “Batch of 89”: A Deep Dive into Recent Rejections
We’ve visualized the key themes and case studies from the Sep 2025 release in the infographic below, which highlights the critical importance of CMC and manufacturing issues.
Real-World Impact: Repercussions of Transparency
The publication of these letters has already sent shockwaves through the biotech sector, illustrating that the FDA’s narrative now travels as fast as a company’s press release.
Case Study 1: Replimune’s RP1 (Vusolimogene Oderparepvec)
- The Scenario: A BLA for an oncolytic immunotherapy in combination with nivolumab for advanced melanoma.
- The Deficiency: The FDA cited heterogeneity of the patient population in the Phase 1/2 “IGNYTE” trial. The Agency concluded the data were not adequate and well-controlled to provide substantial evidence of effectiveness, specifically noting the design might not isolate the contribution of RP1 due to the control arm choices.
- The Lesson: For developers, reliance on single-arm or heterogeneous early-phase data for accelerated pathways is becoming increasingly scrutinized. The FDA is signaling a “return to basics” regarding statistical robustness.
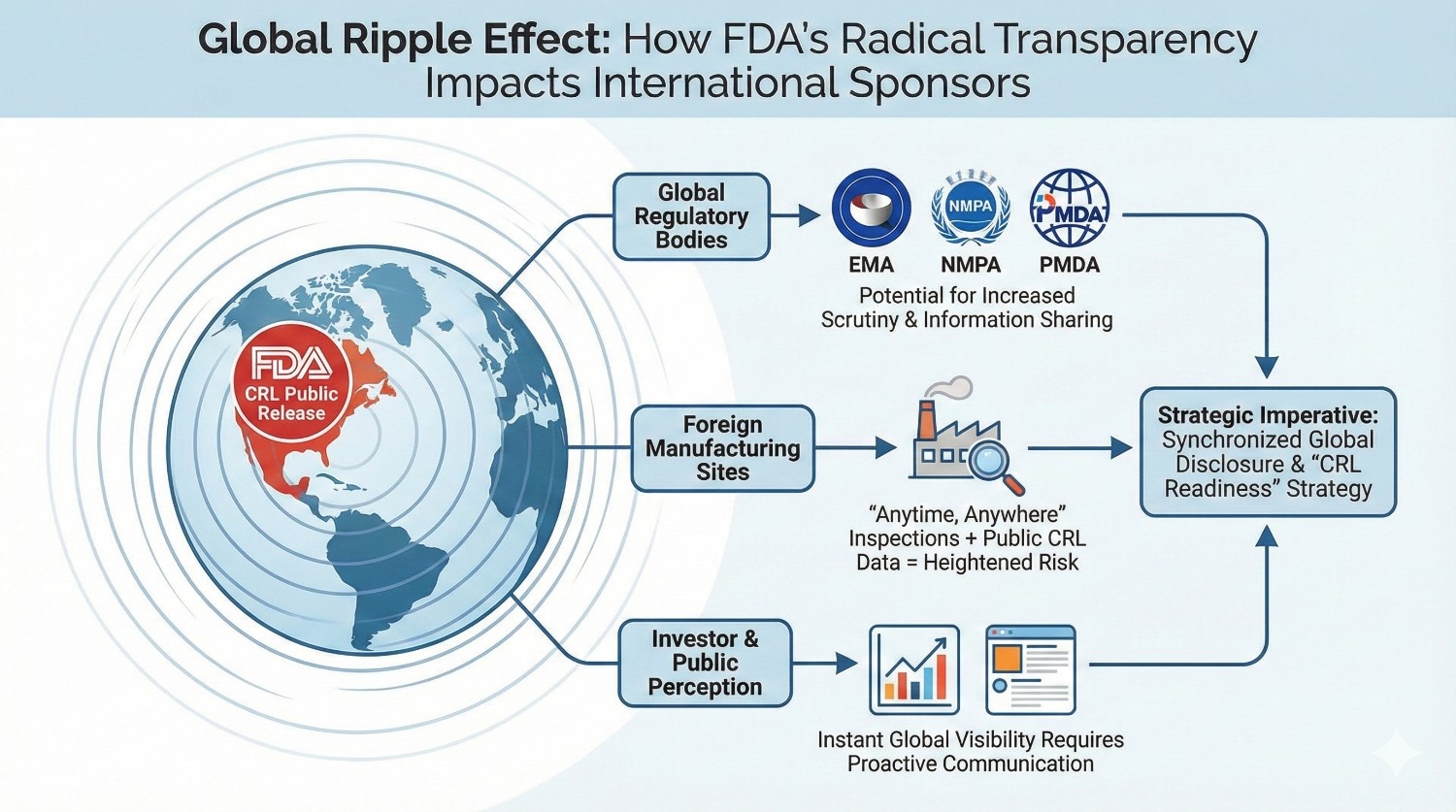
Impact on Foreign Sponsors and International Manufacturers
The infographic below illustrates how a U.S. CRL release can trigger a chain reaction of consequences for foreign sponsors and manufacturers that impact regulatory interactions, manufacturing oversight, and public perception worldwide.
Legal Nuances: The Redaction Battleground
The FDA’s authority to release these letters is derived from the Freedom of Information Act (FOIA) and the FD&C Act, but it remains in tension with 21 CFR 314.430, which prohibits disclosing the existence of an unapproved application. Legal experts predict a court challenge is only a matter of time. Until then, sponsors must be vigilant in identifying what constitutes a Trade Secret (e.g., a specific manufacturing formula) versus the FDA’s Thinking (which the Agency now considers public domain).
Strategic Moves in a Transparent Market
In this glasshouse environment, regulatory and corporate strategy must be perfectly aligned. Stakeholders should consider:
- Auditing Redactions Early: The FDA redacts Trade Secrets (TSI) and Confidential Commercial Information (CCI) on a “best efforts” basis. Sponsors must clearly label proprietary technical data during the submission process to ensure it stays private when the CRL goes live.
- Aligning IR and Regulatory: Investor Relations can no longer “spin” a CRL. If a press release downplays a safety signal that appears unredacted in the CRL database within days, the reputational and legal risk is severe.
- Benchmarking Peers: The openFDA database is now a goldmine for competitive intelligence. Study CRLs in your therapeutic area to identify recurring pitfalls in trial design or endpoint selection before you finalize your own protocols.
- Preparing for “Prompt” Release: If you receive a letter, you should have a response and communication plan ready to go the same day. In the era of radical transparency, the first narrative to hit the market is often the one that sticks.
The Road Ahead: Global Oversight 2026
The FDA isn’t stopping at CRLs. We are seeing a shift toward a system of continuous, global oversight.
- Elsa AI: The FDA’s new generative AI tool is being used to identify high-priority inspection targets by scanning adverse event reports and manufacturing data.
- Unannounced Foreign Inspections: A new standard to hold international facilities to the same rigor as domestic ones, effectively ending the double standard of advance notice.
- Remote Regulatory Assessments (RRAs): Now a permanent fixture for evaluating global compliance across GMP and GCP activities.